Abstract
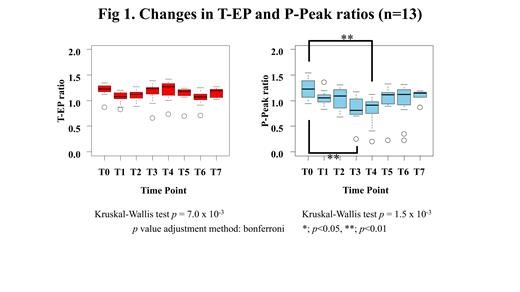
Introduction: Infusion T cells engineering to express CD19-specific chimeric antigen receptor T cells (CAR-T) following lymphodepleting chemotherapy has shown promising efficacy in patients with relapsed and/or refractory CD19-positive B-cell malignancies including B-cell acute lymphoblastic leukemia (B-ALL), and diffuse large B-cell lymphoma (DLBCL). CAR-T therapy can often be associated with cytokine release syndrome (CRS), which has been reported to present severe coagulopathy. Conventional coagulation and fibrinolysis parameters have been reported to worsen in correlation with CRS grading [Hay KA et al. Blood 2017]. In the present study, we hypothesized that the change of balance between coagulation and fibrinolysis due to coagulopathy is associated with CRS after CAR-T therapy. To clarify our hypothesis, we investigated the global coagulation and fibrinolytic function of patients receiving CD19 CAR-T therapy using simultaneous thrombin and plasmin generation assay (T/P-GA). Patients: We enrolled 13 consecutive patients aged 23-69 years (8 males and 5 females, DLBCL; n = 11, B-ALL; n = 2) receiving CD19 CAR-T therapy from May 2020 to December 2020 at a single center in Japan. Due to a history of internal jugular venous thrombosis, one patient received edoxaban, an anticoagulant. Methods: We evaluated the global functions of coagulation and fibrinolysis using T/P-GA [Matsumoto et al. TH 2013]. This clotting was initiated by the mixture of recombinant human tissue factor (Innovin ®, f.c. 1 pM), phospholipid vesicles (f.c. 4 μM), and tissue-type plasminogen activator (f.c. 3.2 nM). We simultaneously monitored thrombin and plasmin generation using thrombin- and plasmin-specific fluorogenic substrate (Z-Gly-Gly-Arg-AMC and BOC-Glu-Lys-Lys-MAC, respectively) in separate microtiter wells. The first derivatives (velocity) of thrombin and plasmin generation were utilized to derive the parameters, lag time (LT), endogenous potential (EP), peak levels (Peak), and time to peak (ttPeak). In this study, EP of thrombin generation (T-EP) and plasmin-peak (P-Peak) were selected as parameters for evaluation. We calculated the ratio of T-EP and P-Peak of patients' plasmas to those of control normal plasma. A ratio > 1.0 was defined as high coagulation or fibrinolytic potential relative to normal. Using fibrinogen (Fbg), D-dimer, and antithrombin (AT), we also monitored the conventional laboratory markers of hemostasis. Soluble IL-2 receptor (sIL-2R) was also measured as a marker of inflammatory cytokines. Day 0 was defined as the day of CAR-T infusion. Plasmas were collected at T0; pre-lymphodepleting chemotherapy, T1; Days -2-0, T2; Days 1-3, T3; Days 4-6, T4; Days 7-9, T5; Days 10-12, T6; Days 13-18, and T7; Days 19-23. Results: All patients had Grade ≤ 2 CRS. Two cases developed CRS-associated coagulopathy, one of whom required fresh frozen plasma transfusion and cryoprecipitate for low Fbg level, and the other required tranexamic acid for hemorrhagic cystitis. The median values of AT remained within the reference value (RV). The median Fbg values were 229-525 mg/dL, and which were significantly greater at T0-T3 than at T5-T7 (p < 0.05). The median D-dimer values were 0.45-3.9 µg/mL, which were within or above the RV with no significant change between time points. The median values of sIL-2R were 819-3,953 U/mL, and which were significantly increased at T3-T4 than at T0-T1 (p < 0.05). T-EP/P-Peak revealed a median of 1,877/21.9, 1,659/18.6, 1,731/19.8, 1,856/15.0, 1,931/16.3, 1,758/18.5, 1,600/19.1, 1,634/20.3, and 1,594/17.4 nM for T0-T7 and control plasma, respectively, indicating the reduced P-Peak ratios (Fig. 1). T-EP ratios showed consistently maintaining > 1 with no significant difference between time points, while P-Peak ratios were significantly lower at T3 and T4 than at T0 (p < 0.01) (median ratios of T-EP/P-peak; T0; 1.23/1.22, T1; 1.08/1.05, T2; 1.12/1.09, T3; 1.23/0.81, T4; 1.27/0.91, T5; 1.19/1.11, T6; 1.07/1.12, and T7; 1.20/1.15, respectively). Conclusion: The results of T/P-GA showed that hemostatic kinetics in patients with Grade ≤ 2 CRS were likely to shift to hypercoagulable states throughout the entire period and that they were likely to experience hypofibrinolytic activity during the CRS phase. These results suggest that the imbalance of coagulation and fibrinolysis might cause organ disorder due to thrombotic tendency associated with CRS after CAR-T therapy.
Takaori-Kondo: Bristol-Myers K.K.: Honoraria; ONO PHARMACEUTICAL CO., LTD.: Research Funding; Celgene: Research Funding. Nogami: Chugai Pharmaceutical Co., Ltd.: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal